Control over culture media with MiStraCon® strains
29 de November de 2023Scharlau MiStraCon® strains from the American Type Culture Collection (ATCC®) include the most commonly used strains in the food industry, pharmaceutical industry, water quality control and clinical settings.
Scharlab is attached to the ATCC® licensed derivatives programme. Thus, the ATCC Licensed Derivative mark establishes that Scharlab S.L. is licensed to use these trademarks and sell products derived from ATCC® cultures.
All MiStraCon® products are supplied with detailed instructions for use and have a certificate of analysis that guarantees their authenticity, traceability and the number of subcultures produced from the original reference culture.

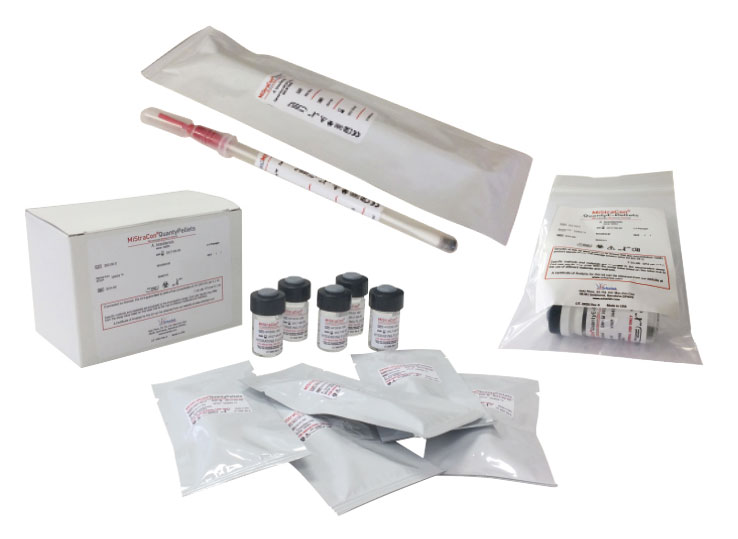
Scharlau MiStraCon® strains: three product formats
This line of Scharlau products consists of three product formats:
MiStraCon®Swabs2
Unquantified bacterial strains in freeze-dried pellet prepared for self-hydration and for direct use by means of a swab incorporated in the device.
MiStraCon®QuantyPellets
Quantified bacterial strains in lyophilised pellet ready to hydrate and use in growth promotion assays of culture media. Commonly used in the pharmaceutical industry.
MiStraCon®QuantyE-Pellets
Quantified bacterial strains presented in a lyophilised pellet ready to hydrate and prepare quantified dilutions.
MiStraCon®Swabs2 format
| Format | Packaging | Content | CFU | Applications/Specifications |
MiStraCon®Swabs2 | Each unit contains a lyophilized pellet, moisturizing fluid and inoculation swab, all sealed in an aluminized envelope | 2 units of MiStraCon®Swabs2 | Not quantified | Culture purposes. Daily QC- QC of identification systems and assay kits. QC of antimicrobial susceptibility test. Verification and validation. |
MiStraCon®QuantyPellets and MiStraCon®QuantyE-Pellets formats
| Format | Packaging | Content | CFU | Applications/Specifications |
MiStraCon®QuantyPellets | 1 box. Each box contains 5 vials of a single quantified microorganism and 5 vials of hydrating fluid. | 5 vials with 1 quantified pellet (1 pellet/vial) and 5 vials of hydrating fluid (1.2 mL) | <100 by 0.1 mL when processed according to directions | Growth promotion testing, Media challenge testing, Suitability of counting methods, Suitability of sterility tests, Suitability of tests for specified microorganisms. Microbial limits testing. Microbial enumeration testing. Validation of neutralization methods. Methods requiring a low CFU concentration. |
MiStraCon®QuantyE-Pellets | 1 vial of lyophilized pallets in a plastic bag with hermetic seal | 1 vial with 5 pellets | 1,0E+03 – 1,0E+07 per pellet | Detection and enumeration methods. Verification/Validation. Bioburden determination. Minimal lethal concentration. Disinfectant qualification. Water tests. Proficiency tests. Methods requiring a specific CFU range. |
For further information or to enquire about other products or services, please write to helpdesk@scharlab.com.

 Rest of the World
Rest of the World




