Letheen Modified Agar
01-237
ASTM / BAM / FDA / ISO / USP
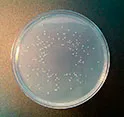
Solid medium for the primary screening of microorganisms in cosmetics according to the FDA and ISO 21149, 22717 and 22718 standards. Needs Polisorbate 80 additive (Art. No.:TW0080).
ASTM / BAM / FDA / ISO / USP
Solid medium for the primary screening of microorganisms in cosmetics according to the FDA and ISO 21149, 22717 and 22718 standards. Needs Polisorbate 80 additive (Art. No.:TW0080).

01-237
ASTM / BAM / FDA / ISO / USP
Solid medium for the primary screening of microorganisms in cosmetics according to the FDA and ISO 21149, 22717 and 22718 standards. Needs Polisorbate 80 additive (Art. No.:TW0080).
Features
+See More

 Rest of the World
Rest of the World






